SYSA200_L
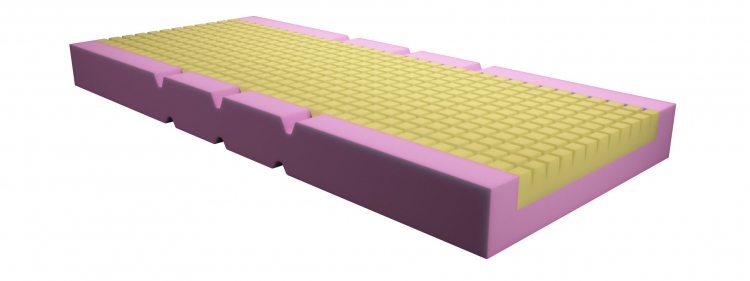
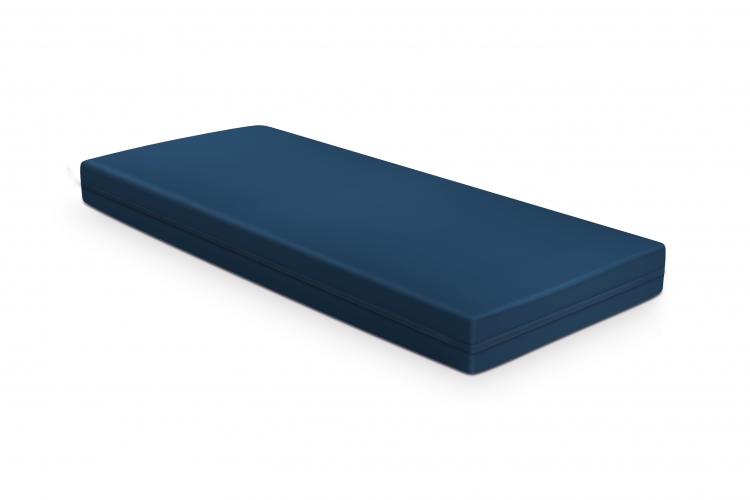
Mattress Synstatù Antidecubitus Sycura, two modules, with pyramid-shaped heat and pressure formable surface made of Synergel®, for specialized decubitus risk patients. Inclusive of cover. Dim. cm 195x85x14 h
Features
Browse Catalogue
Technical Data Sheet
Ask for information
Features
Extremely high-performance two-module static mattress in open-cell polyurethane foam with a heat and pressure formable, truncated pyramid-shaped support surface made of Synergelù. For general and specialized patients and those with a low to medium-high decubitus risk. The mattress is equipped with a special cover, waterproof, breathable, bielastic, antistatic, treated with Zeneca bacteriostatic and fungistatic system. Recommended for a highly breathable posture with a low pressure gradient on the musculoskeletal system, and thus the prevention of pressure-induced skin lesions and the management of pain and inflammatory symptoms. Indicated for incontinent patients and for a better epidemiological control. Medical device bearing the CE mark, performance standards certified by UNI 10707:2003, certified for the comfort and antidecubitus index (Ergocert D28) and certified in fire resistance class 1 IM. Safe working load from 30 kg up to 250 kg, sag factor not applicable. Cover colour: blue. Latex-free product. Radiotranslucent product.
Two-module mattress with one-piece structure (with incisions at bending points) and one-piece cover. The base module (8 cm thick) has a cradle structure with containment edges (height 14 cm, width 7.5 cm) made of non-deformable polyurethane foam of the polyether 40S type with a high density (40 Kg/m3) and high bearing capacity (5.2 Kpa), so as to allow cardiac massage, forced postures and to provide adequate support and safety for the patient.
The antidecubitus support module is made of Synergelù (density 60 Kg/m3 - bearing capacity 3.6 Kpa - 8 cm thick), a special copolymer foam made of polyether, polyester and silicone, flexible, shapable, heat and pressure formable and with an elastic response (resilience) that cannot be measured in accordance with the UNI standards. Sag factor not applicable. The lack of shape memory and the special shape ensure a drastic reduction in both vertical and tangential (friction) compression on the tissues, optimum posture and comfort for all types of patient, effective prevention for low and medium-high risk patients and treatment for stage 1 decubitus ulcers. Water vapour permeability (breathability) d3/s 1.5 ù 5% (UNI EN ISO 7231 lab. test indicative measures.)
All the materials of which the padding is made have an open-cell structure to ensure high breathability. Both the padding and the cover are treated and certified with proper bacteriostatic and fungistatic sanitary system for the patient's hygienic protection.
The cover is in Sycura, blue-coloured, a special coated fabric (150 g/m2) consisting of a microporous polyurethane membrane and an internal support made of polyester mesh. The Sycuraù cover is waterproof and impermeable to biological fluids, breathable (water vapour permeable > 300 g/m2 in 24 hours UNI 4818.26), bielastic to avoid hammock effect, antistatic (prevents from the imbalance of electric charges), self-extinguishing, treated and certified with Zeneca bacteriostatic and fungistatic sanitary system, washable and decontaminable in washing machine or autoclave. The Sycuraù cover is a full encasement with darts on the corners and is provided on one of the long sides and the two short sides with a sturdy black-coloured nylon zipper with stainless slider (chain and slider nr.6 YKK).
The non-deformability and durability standards of the Synstatù Antidecubitus Sycura mattress are certified at the strictest level (45,000 cycles) by a leading testing and certification laboratory in accordance with the UNI 10707 standard, rev. 2003 and the comfort and the antidecubitus index are certified in accordance with the ErgoCert D28 technical regulation.
The Synstatù Antidecubitus Sycuraù mattress bears the CE mark as laid down by the European directive 93/42/EEC on Medical Devices and is certified in fire resistance class 1 IM by the Ministry of the Interior in accordance with the CSE RF 4/83 standard laid down in MD of 26/06/84 (UNI 9175 and UNI 9175/FA1 laid down in MD of 03/09/2001).
The Synstatù Antidecubitus Sycuraù mattress is indicated for use as a primary prevention device for patients with a low and medium risk of developing decubitus ulcers (grades 4 and 3 on the Norton scale with a score of over 18 to 14). It is useful when combined with the clinical and surgical practices for grade 2 patients (score of 10-14 on the Norton scale). The Synstatù Antidecubitus Sycuraù mattress is indicated for the treatment of stage 1 decubitus ulcers and with greater caution for the treatment of stage 2 decubitus ulcers in combination with the current nursing, pharmacological and surgical procedures.
Instructions for use: the Synstatù series mattress is to use with the support surface corresponding to the truncated pyramid-shaped surface of the padding in Synergelù 35S (ecru colour), as written on the label, with the instructions, situated oppositely to the support surface and attached to the cover of the product.
The wrong use does not cause damage and does not compromise safety but it only does not allow for the optimal use of the product.
Registration on the Medical Devices Repertoire nr. 18856 - CND - GMND code: Y033306 - 35228
ISO code: 03.33.06.006
Weight 11.7 kg
Two-module mattress with one-piece structure (with incisions at bending points) and one-piece cover. The base module (8 cm thick) has a cradle structure with containment edges (height 14 cm, width 7.5 cm) made of non-deformable polyurethane foam of the polyether 40S type with a high density (40 Kg/m3) and high bearing capacity (5.2 Kpa), so as to allow cardiac massage, forced postures and to provide adequate support and safety for the patient.
The antidecubitus support module is made of Synergelù (density 60 Kg/m3 - bearing capacity 3.6 Kpa - 8 cm thick), a special copolymer foam made of polyether, polyester and silicone, flexible, shapable, heat and pressure formable and with an elastic response (resilience) that cannot be measured in accordance with the UNI standards. Sag factor not applicable. The lack of shape memory and the special shape ensure a drastic reduction in both vertical and tangential (friction) compression on the tissues, optimum posture and comfort for all types of patient, effective prevention for low and medium-high risk patients and treatment for stage 1 decubitus ulcers. Water vapour permeability (breathability) d3/s 1.5 ù 5% (UNI EN ISO 7231 lab. test indicative measures.)
All the materials of which the padding is made have an open-cell structure to ensure high breathability. Both the padding and the cover are treated and certified with proper bacteriostatic and fungistatic sanitary system for the patient's hygienic protection.
The cover is in Sycura, blue-coloured, a special coated fabric (150 g/m2) consisting of a microporous polyurethane membrane and an internal support made of polyester mesh. The Sycuraù cover is waterproof and impermeable to biological fluids, breathable (water vapour permeable > 300 g/m2 in 24 hours UNI 4818.26), bielastic to avoid hammock effect, antistatic (prevents from the imbalance of electric charges), self-extinguishing, treated and certified with Zeneca bacteriostatic and fungistatic sanitary system, washable and decontaminable in washing machine or autoclave. The Sycuraù cover is a full encasement with darts on the corners and is provided on one of the long sides and the two short sides with a sturdy black-coloured nylon zipper with stainless slider (chain and slider nr.6 YKK).
The non-deformability and durability standards of the Synstatù Antidecubitus Sycura mattress are certified at the strictest level (45,000 cycles) by a leading testing and certification laboratory in accordance with the UNI 10707 standard, rev. 2003 and the comfort and the antidecubitus index are certified in accordance with the ErgoCert D28 technical regulation.
The Synstatù Antidecubitus Sycuraù mattress bears the CE mark as laid down by the European directive 93/42/EEC on Medical Devices and is certified in fire resistance class 1 IM by the Ministry of the Interior in accordance with the CSE RF 4/83 standard laid down in MD of 26/06/84 (UNI 9175 and UNI 9175/FA1 laid down in MD of 03/09/2001).
The Synstatù Antidecubitus Sycuraù mattress is indicated for use as a primary prevention device for patients with a low and medium risk of developing decubitus ulcers (grades 4 and 3 on the Norton scale with a score of over 18 to 14). It is useful when combined with the clinical and surgical practices for grade 2 patients (score of 10-14 on the Norton scale). The Synstatù Antidecubitus Sycuraù mattress is indicated for the treatment of stage 1 decubitus ulcers and with greater caution for the treatment of stage 2 decubitus ulcers in combination with the current nursing, pharmacological and surgical procedures.
Instructions for use: the Synstatù series mattress is to use with the support surface corresponding to the truncated pyramid-shaped surface of the padding in Synergelù 35S (ecru colour), as written on the label, with the instructions, situated oppositely to the support surface and attached to the cover of the product.
The wrong use does not cause damage and does not compromise safety but it only does not allow for the optimal use of the product.
Registration on the Medical Devices Repertoire nr. 18856 - CND - GMND code: Y033306 - 35228
ISO code: 03.33.06.006
Weight 11.7 kg
Most Viewed

SILVER Relaxing armchair, reclining back and legrest, simultaneous movement
cod. 376445

Range 300 bedside cabinets
cod. 330600

INTERgo TRANSPORTATION AND STORAGE ELEMENTS
cod. 326500LC